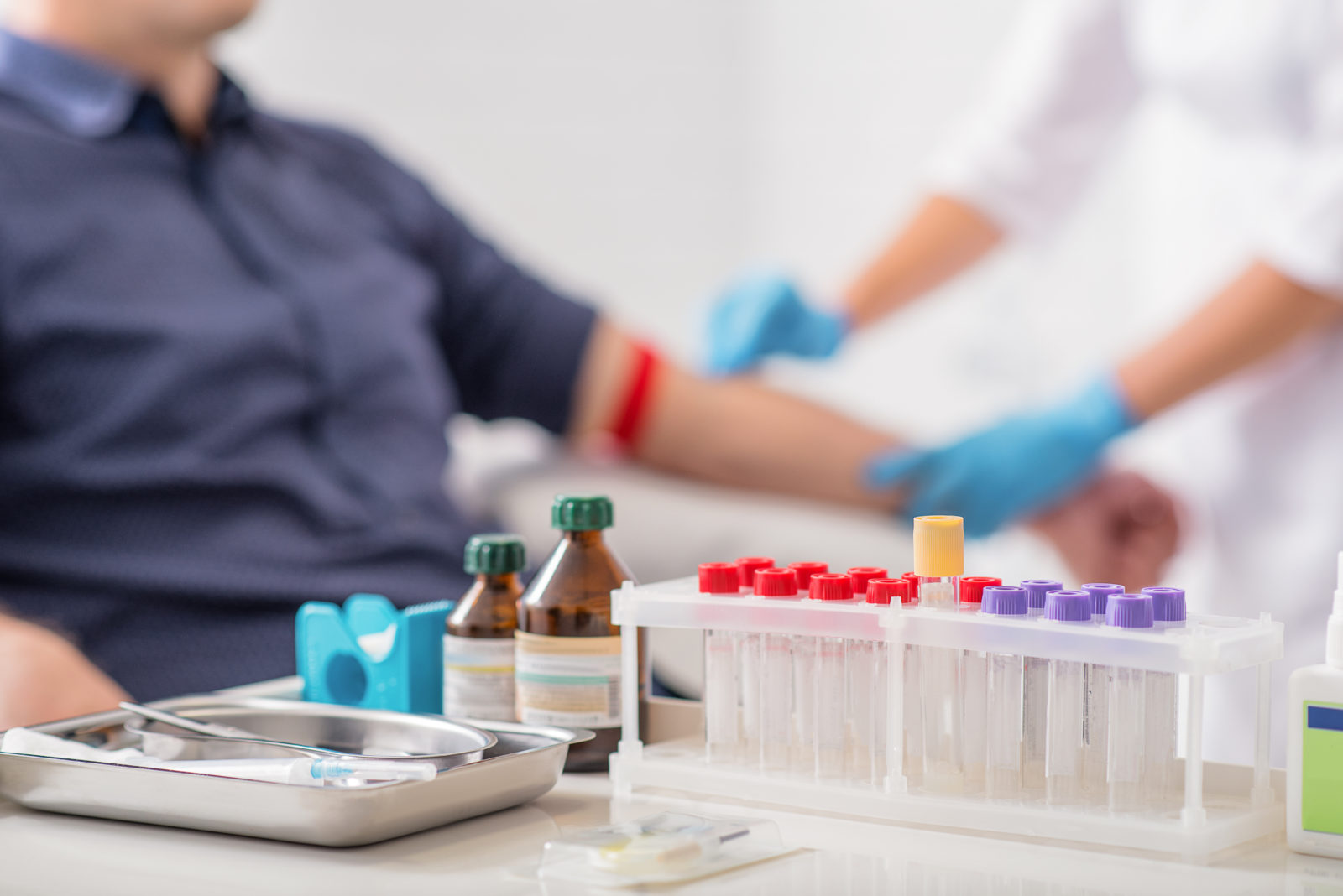
A SIMPLE blood test that detects bowel cancer earlier – and improves the chances of survival for thousands of patients is one step closer to being available on the NHS, after showing excellent results in a primary care trial.
The Raman Spectrometry (RS) blood test has been developed by researchers at Swansea University led by Professors Dean Harris and Peter Dunstan, with funding from Cancer Research Wales and Health and Care Research Wales.
Support from Life Sciences Hub Wales has now enabled Welsh start-up business ‘CanSense’ to secure an additional £1.2 million from the National Institute for Health and Care Research (NIHR) to further develop the blood test to use in clinical practice across Wales.
Results from the ground-breaking study involving 27 practices and 595 patients across West Wales showed 79 per cent of early-stage bowel cancers and 100 per cent of advanced bowel cancers were picked up by the test.
Early comparisons with other tests currently available in primary care have shown the RS blood test to have greater sensitivity for the detection of bowel cancer.
It is hoped the non-invasive test will dramatically cut waiting times for diagnosis and reduce the need for unnecessary invasive procedures such as colonoscopies.
Bowel cancer is currently the second leading cause of cancer death in Wales, with waiting times for diagnosis and treatment some of the longest in the developed world.
Sadly, many of the 2,200 diagnosed cases in Wales each year are detected at an advanced stage, like Lynda, 66, from Swansea.
She said: “I was diagnosed with bowel cancer in June of 2021. I went to the GP with a small amount of blood in my stool and I was referred to the hospital for investigation.
“I didn’t have many symptoms. I am a very fit and active person, so I thought my weight loss was just down to being very physically active in the pool, gym, and on my bike.
“It came as a huge shock to me to find out I had 12cm of cancer in my bowel – and I needed immediate treatment to save my life.”
Lynda – who now has a stoma and is cancer free – added: “I believe by introducing blood tests like this we can save many more lives in the future and prevent late diagnosis of bowel cancer in the majority of cases.”
Currently, a high number of unnecessary colonoscopies are conducted in Wales to ensure cases of bowel cancer are detected.
Since the start of the pandemic, waiting lists for the procedure have significantly increased – with just under 8000 patients currently waiting to be screened. Half of these have waited longer than three months.
These delays can reduce the chances of patient survival, with many people being diagnosed too late.
The blood test CanSense is developing hopes to change all that by providing accurate results within 48 hours, preventing unnecessary colonoscopies and relieving pressure on the NHS.
Dr Cerys Jenkins is Co-founder and Director at CanSense. Her research into Raman technology began eight years ago when she was funded by Cancer Research Wales to complete a PhD at Swansea University.
Cerys – whose grandfather had bowel cancer – said: “The goal for this research has always been to translate it into something that fits into the existing patient pathway. Having this test available at the triage stage would save time, money, but most importantly save the patients from anxiety and unnecessary diagnostic tests.
“My grandfather was diagnosed with bowel cancer 30 years ago, and he said the colonoscopy was the most humiliating experience of his life. We haven’t moved forward from this state of play in three decades. And although we would never seek to replace colonoscopies as the gold standard in diagnostics, the Raman test could become an important tool in improving health outcomes across Wales.”
Speaking on their involvement in the project, Ann Tate, CEO Cancer Research Wales, said: ““As a charity, investing heavily in research to improve cancer diagnosis is part of our DNA. All cancer patients in Wales deserve innovative treatments for a better future and this test provides a solution for clinicians to diagnose cancer at an earlier stage. Our contribution was only possible due to the support of people across Wales, and for that we are extremely thankful.”
Funding secured via the UK’s NIHR with the support of Life Sciences Hub Wales will now allow the research team at Swansea to develop the test into a certified format to use in GP surgeries across the UK.
The £1.2 million Invention for Innovation grant provides a roadmap for regulatory approval and product development to allow the test to be used within the NHS within 2 years.
Adam Bryant, CEO and Business Founder of CanSense, said: “For the past eight years, our team has dedicated itself to creating something that we believe is as exciting as it is revolutionary. We now have a proven, rapid, and affordable test that can save thousands of lives. With continued collaboration and funding, we now have a clear path to making this test available to GPs in Wales and beyond.”
Commenting on Life Sciences Hub Wales’ role in securing the funding, Cari-Anne Quinn, CEO, said: “Earlier diagnosis and detection of cancer using less invasive methods is an essential part of the preventative agenda to make healthcare more efficient and improve patient outcomes. Life Sciences Hub Wales is delighted to be involved in this project through our funding support services and look forward to seeing its impact across Wales.”








Leave a Reply
View Comments